Models of Infectious Diseases (BSL-3)
- Postdoctoral fellow:
The Unit of Models of Infectious Diseases offers specialized services for advanced research and preclinical testing of infectious diseases. Operating under strict biosafety level 3 (BSL-3) conditions, our facility is among the largest and most advanced in the Czech Republic, with a capacity of over 500 cages. We support both in vivo and in vitro research on a wide range of human and animal pathogens, including viruses, bacteria, yeasts, and protozoa. Our expertise covers studies of both acute and chronic phases of infection, leveraging deep knowledge in animal physiology, pathology, and related fields. The facility is fully equipped for the development and testing of new therapeutic and prophylactic interventions. By adhering to rigorous BSL-3 standards, we ensure the highest level of safety for our team and the wider community, enabling cutting-edge research with dangerous pathogens.
Standard services Infectious disease research
We offer various models of viral and bacterial infections for in vivo experiments in mice such as SARS-CoV-2, Hepatitis B, Acinetobacter baumanii orMonkeypox. We work with AAV and lentiviral vectors for targeted gene delivery. Advanced animal research is conducted by highly trained specialists, caretakers, and technicians, proficient in both basic and advanced invasive and non-invasive techniques. We have established and validated methodologies covering pathogen or substance delivery, sample collection, and specialized animal treatment.
State-of-the-art in vivo and in vitro research is supported by cutting-edge technology and collaboration with experts in related fields, enabling comprehensive and specialized scientific research. Our facility designs tailored research projects and performs biochemical, immunological, and metabolic analyses to assess disease impact. We also conduct comparative functional assays, including lung function, cardiac function, and metabolic alterations.
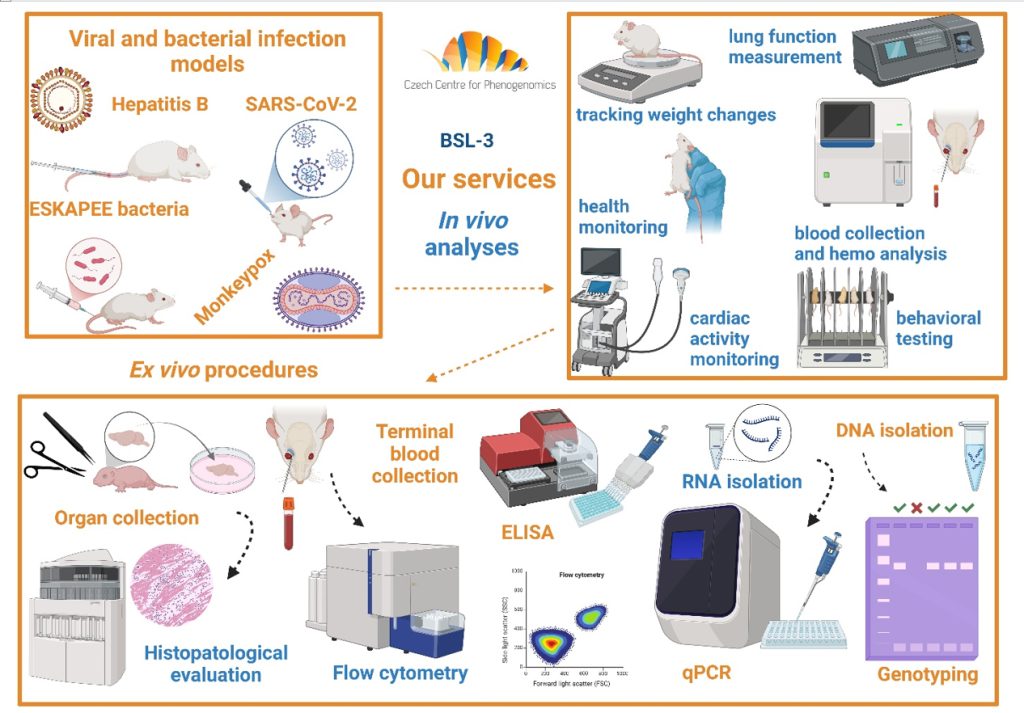
Animal Biosafety Level 3 facility – fully equipped and staffed facility for work on infectious diseases.
Infectious diseases – we are able to establish and work with different infectious agents.
Molecular analyses – Methods involving the separation of RNA, DNA, or proteins from a variety of tissues, followed by analysis utilizing methods such as ELISA, PCR, RT-qPCR, or Western Blots.
Evaluation and Reporting – After statistical evaluation from our bioinformatics team we deliver coherent report including conclusions and discussion per the requestor’s needs.
Advanced Phenotyping in Infection
Through close collaboration with other CCP units, we offer comprehensive services, including preclinical pharmacokinetics and toxicity studies, metabolic and immunological analyses, physiological and pathological evaluations, and advanced histological examinations. This integrative approach enables detailed characterization and efficient evaluation of therapeutic interventions against infectious diseases.
Lung function – Complex respiratory analysis and challanges and administrations relevant to the respiratory pathways.
Immunology – An in-depth investigation of the immunological state of animals reflecting the infectious state, comprising a comprehensive flow cytometry panel with adjustments based on individual requests.
Biochemistry – Encompasses a wide range of methodologies, such as hematoanalysis, urinalysis, and the evaluation of clinical chemistry markers.
Metabolism – Conditioning animals with a diet heavy in fat or evaluating various metabolites, such as glucose tolerance tests to evaluate diabetic stages.
Cardiovascular – Non-invasiveEchocardiography for compolex heart function assessment, including other essential markers such as oxygen saturation
Behavioral evaluation – Includes advanced modalities such as rotarod or grip strength tests to evaluate motor function, learning ability, and memory. The basic SHIRPA evaluation is used to monitor and quantify animal wellbeing.
Bioimaging – A whole-body imager system with bioluminiscence detection capabilities that is available for anatomical viewing and signal analysis. Optical microscopy for in vitro assays.
Histopathological analysis – Comprehensive examination by qualified pathologists, including the utilization of cutting-edge immunohistochemical staining techniques based on individual requirements of projects.
Currently estabilished infectious models
SARS-CoV-2 strains
Transgenic or AAV senzitised mice expressing hACE2 ubiquitously or locally. Infection progression depends on particular model, dose, route of infection and viral strain used. Available SARS-CoV-2 viral strains include:
- B.1
- B.1.351 Beta
- B.1.1.529 Omicron
- BA.5
- XBB.1.5.12 Omicron Kraken
Acinetobacter baumannii
Type strain ATCC 19606. Pulmonary infection of immunocompetent mice is achieved by intratracheal administration in immunocompetent mice. Additionally, systemic infection can be reached in transiently immunocompromised animals. Suitable for testing antibiotic or antibacterial treatments.
Hepatitis B virus, genotype D and A
AAV-HBV vector is administered intravenously to transfect hepatocytes and cause stable chronic replicative infection lasting for over three months. Hepatitis B surface antigen (HBsAg) and Hepatitis B envelope antigen (HBeAg) as well as viral titer is stable through the course of the infection. The model is responsive to treatment regimens and is suitable to test antivirals.
Custom services Contract research projects
We offer end-to-end design and execution of research projects tailored to your specific scientific objectives. Our team collaborates closely with clients to develop experimental protocols, manage project timelines, and deliver high-quality, reliable results.
Custom services Custom assay development
Our experts can develop and validate bespoke assays for pathogen detection, quantification, or drug screening. Whether you need molecular, immunological, or cell-based assays, we ensure sensitivity, specificity, and reproducibility to meet your research needs.
Custom services Preclinical testing of therapeutics and vaccines
Our facility is equipped for rigorous preclinical evaluation of new drugs, biologics, and vaccines under BSL-3 conditions. We assess efficacy, safety, and pharmacokinetics in relevant animal models to support your translational research.
Custom services Introduction of new infectious diseases
We provide comprehensive support in acquiring legal licenses required for the introduction of new infectious pathogens, genetically modified organisms, or project-specific experimental procedures. We ensure full compliance with national and international regulations.
Custom services Custom animal model generation
In our laboratory, we can design and prepare genetically modified mice that are sensitive to infection by various pathogens.
- Standard and infection-sensitized mouse models
- Customized introduction and management of new infectious disease models
- Surveillance and detailed analysis of disease progression and treatment responses
- Models with pre-existing medical conditions such as obesity, cancer, or immunocompromised states
- Organ-specific infection models (whole organism, intestine, heart, brain)
Technology platforms
Unit of Models of Infectious Diseases is being upgraded from the OP JAC project CZ.02.01.01/00/23_015/0008189 Upgrade of the large research infrastructure CCP III.