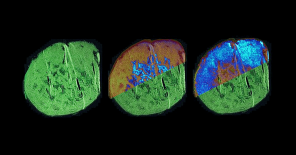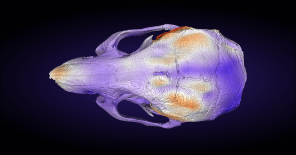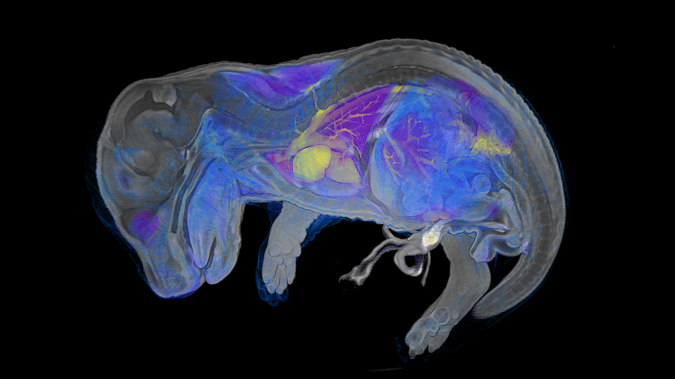
Phenotyping
The phenotyping module at the Czech Center for Phenogenomics houses a comprehensive collection of tools for the physiological
and morphological assessment of experimental mice and rats
in a controlled SPF (specific pathogen-free) environment.
Our mission is to support the preclinical research and development community with service of the highest professional standard.
More info
Our experienced staff offers a wide variety of standardized tests and services, including those of IMPReSS (International Mouse Phenotyping Resource of Standardised Screens), mandated by our active partnership in the International Mouse Phenotyping Consortium. Notable is our capacity for conducting comprehensive phenotyping pipelines, providing a wide breadth of clinical information per experimental animal, and thereby minimizing overall animal usage.